Prof. Muller’s independent career of over 30 years has been distinguished by many outstanding scientific discoveries and contributions. He is an established leader of the international breast cancer research community and a cornerstone of McGill’s cancer research and training programs who has helped to launch and support the careers of many scientists and clinicians. This legacy continues to have a major impact on breast cancer research and care.
Above all, however, Prof. Muller is a trailblazer who was instrumental in establishing the field of genetically engineered mouse models (GEMMs) of breast cancer. These vital tools, shared widely by Prof. Muller with colleagues around the world, have proven to be indispensable for understanding breast cancer at the molecular, cellular and organismal levels. They have been a major factor in the development of breast cancer therapies, from targeted therapies against driving oncogenes to the latest immunotherapies, and continue to be relied on for cutting-edge translational studies by Prof. Muller and many others.
Prof. William J. Muller completed his Ph.D in 1986 at McMaster University, in the group of Prof. John Hassell. His original research focus was on tumor virology, where he made important contributions to our understanding of the genetic elements and mechanisms regulating DNA replication and gene expression in polyomaviruses.
Following his graduation, Prof. Muller developed a strong interest in developing transgenic mice as models of cancer, a field which at that time was at its very beginning. He had the foresight to join the group of Prof. Philip Leder at Harvard, one of the epicentres of this rapidly emerging research area, with a major focus on adapting a genetic element known as the mouse mammary tumor virus long terminal repeat (MMTV-LTR) to drive transgene expression in the mouse mammary epithelium. These efforts led to the development of the first GEMM of breast cancer (the MMTV-H-Ras model) in the Leder group. By allowing the transformation of genetically normal cells to be studied in an intact tissue environment, as opposed to the prevailing models of the time that used immortalized cell lines grown under artificial culture conditions, these early GEMMs were a true test of the transforming nature of putative oncogenes.
With his remarkable aptitude for molecular biology and genetics, Prof. Muller had an immediate impact on the development of some of the original GEMMs of mammary carcinogenesis. He made key contributions to a highly influential study that discovered in vivo cooperation between the oncogenes H-Ras and c-Myc.
He then led the development of the first GEMM expressing the clinically relevant breast cancer oncogene ERBB2/HER2, described in a landmark paper published in Cell. While clinical and in vitro observations had linked genomic amplification of ERBB2 with breast cancer, Prof. Muller’s demonstration that ERBB2 overexpression was sufficient to cause breast cancer was definitive proof that ERBB2 is a breast cancer oncogene. These and Prof. Muller’s subsequent models were vital steps enabling many translational studies that led to widely successful targeted therapies for treatment of HER2+ breast cancer.
Throughout his career as an independent investigator, Prof. Muller has constantly been at the forefront of breast cancer research. Building on his post-doctoral research, Prof. Muller’s ERBB2-driven GEMMs of breast cancer have followed an iterative process of continual refinement, always incorporating the latest technological advances. In a series of studies spanning two decades, his approach has yielded many models recapitulating key features of the human disease. These include the only GEMM that expresses oncogenic ErbB2 under the transcriptional control of its endogenous promoter, a model that also spontaneously amplifies the ErbB2 locus, including adjacent genes, in the exact manner observed in HER2+ breast cancer patients (Andrechek et al, PNAS, 2001).
While he is well known for his development of ErbB2-driven GEMMs, the MMTV-PyV mT (polyomavirus middle-T antigen) model is also among Prof. Muller’s most significant accomplishments (Guy et al., Mol. Cell. Biol., 2002). Mammary tumors develop in this model with a stepwise progression remarkably like that observed in human patients, leading to the emergence of invasive, metastatic breast cancer with complete penetrance. The MMTV-PyV mT model quickly became one of the most important GEMMs of breast cancer and continues to be widely used today, particularly for studies of metastasis and the role of the immune system in breast cancer.
Prof. Muller remains an innovator in the design of breast cancer GEMMs that reflect important clinical observations. For example, he has recently established a series of models expressing mutant forms of PIK3CA and ESR1 (estrogen receptor alpha) that were found as driver of transformation, metastatic progression and endocrine therapy resistance in human patients.
Beyond simply developing models, Prof. Muller has combined GEMMs with other approaches, including cell-based and patient-derived models as well as analysis of clinical samples, to gain unique insight into breast carcinogenesis. Among Prof. Muller’s most fascinating and significant findings was the discovery that mammary tumors in GEMMs overexpressing wild-type ErbB2 developed frequent, spontaneous mutations affecting a specific extracellular region of the ErbB2 protein. This created a constitutively active form of ErbB2 remarkably like a naturally occurring splice variant.
With collaborators from Foundation Medicine, Prof. Muller recently discovered a novel class of ERBB2 mutations that constitutively generates this potent oncogenic splice variant of ERBB2 (Smith et al., PNAS, 2020), a finding that may expand the use of targeted therapies against this key breast cancer oncogene.
Prof. Muller was also an early adopter of conditional gene targeting strategies to investigate key determinants of mammary tumorigenesis and prominent drug targets, including kinases such as c-Src (Marcotte, Smith et al., PNAS, 2012), Akt (Hutchinson et al., Cancer Res., 2004; Dillon et al., Cancer Res., 2009) and integrin-linked kinase (ILK) (White et al., Oncogene 2001; Huck et al. Oncogene, 2010), adaptor proteins such as ShcA (Ursini-Siegel et al., EMBO J. 2008), transcriptional regulators such as STAT3 (Ranger et al., Cancer Res. 2009) and EZH2 (Smith et al., Nat. Comms., 2019; Hirukawa et al., Nat. Comms, 2018) and tumor suppressors including p53 (Li et al., Mol. Cell Bio., 2007), PTEN (Dourdin et al. Cancer Res., 2008) and 14-3-3σ (Ling et al., Genes & Dev., 2010; Ling et al., Cancer Discovery, 2011).
Prof. Muller’s discoveries have had a significant impact on our knowledge of all major aspects of breast cancer biology, including key oncogene-associated signaling pathways, cell adhesion, angiogenesis, epigenetics, and metabolism, leading to new therapeutic strategies for hard-to-treat, metastatic breast cancers. Importantly, the nature of Prof. Muller’s unique in vivo models, with their complete tumor microenvironment including a fully functional immune system, has also enabled their extensive use by Prof. Muller and others for studying the role of stromal cells, including immune cell populations, in tumor progression (DeNardo et al., Cancer Cell, 2009; Lin et al., Cancer Res, 2006; Malanchi et al., Nature 2011).
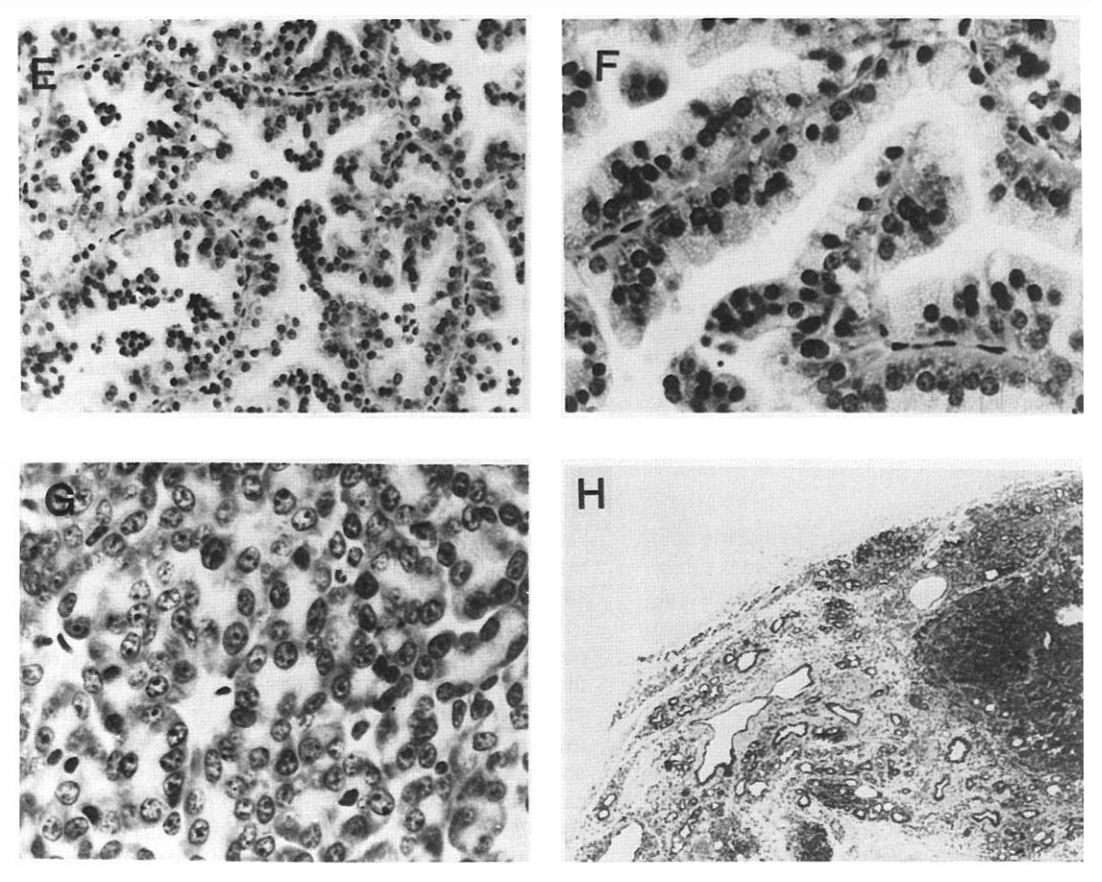
From "Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: Synergistic action of oncogenes in vivo"
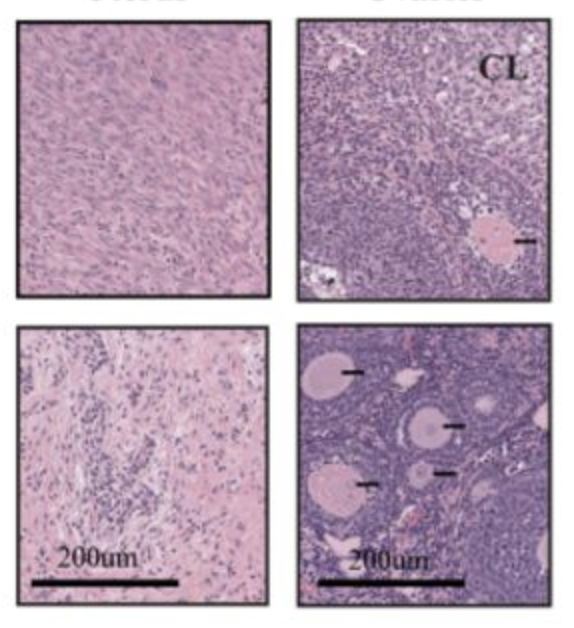
From "Point-activated ESR1Y541S has a dramatic effect on the development of sexually dimorphic organs"
In addition, Prof. Muller has been recognized by his peers through prestigious awards at each stage of his career, including an MRC Scientist Award from the Medical Research Council of Canada, a Tier 1 Canada Research Chair in Molecular Oncology, and appointment as a Fellow of the Royal Society of Canada in 2011.
Prof. Muller is a strong advocate for the use of powerful, clinically relevant models to advance breast cancer research and he is well-known and widely respected throughout the global breast cancer research community. This includes both academia and industry, where Prof. Muller’s models have been widely used by pharmaceutical and biotech companies to investigate novel therapies, including immunotherapies. He is extremely collaborative, forming a wide range of strong working relationships, including with junior investigators, helping them to advance their careers through providing access to unique resources and assistance with publishing and funding their work as well as advice and mentorship.
A major aspect of Prof. Muller’s outreach is his willingness to share models, reagents and resources created in his lab, including prior to their publication, with colleagues around the world. This has had a clear and remarkable impact on the entire cancer research field. Models created by Prof. Muller continue to facilitate cutting-edge studies of cancer, including physiological studies of tumor immunology and studies of metastasis in a context where all the steps of the metastatic cascade are involved.
In summary, William J. Muller has had an exemplary career filled with major accomplishments in the fields of molecular oncology, biochemistry and genetics, focused on breast cancer. He continues to have an impact through his significant discoveries, powerful experimental models that help the entire community address the most important questions, and outstanding mentorship.
Discovery of a Cancer Biomarker by Researchers of the Rosalind & Morris Goodman Cancer Institute