The Metabolomics Innovation
Resource (MIR), consists
of a large array of platforms
managed by professional staff
and in collaboration with
the Oncometabolism
research team.
This facility is always under development improving the breadth of metabolites we can measure thanks to generous grants from the Terry Fox Foundation, Canadian Foundation for Innovation, Génome Québec, and a donation from the Dr. John R. Fraser and Mrs. Clara M. Fraser Memorial Trust.
Metabolomics is one of the emerging “omic” sciences in systems biology that include genomics, proteomics and transcriptomics, providing a greater holistic view of a system’s biological state. Metabolomics is the comprehensive analysis of metabolites (up to 2000 Da) that are either metabolic pathway intermediates or endpoints. An understanding of the biochemical changes in metabolism between healthy and disease states will potentially lead to better models for the disease progression, (dis)regulation, diagnosis, prognosis, treatment and response to treatment.
Platform Manager: Daina Avizonis
Tel: 514-398-5199
Lab: 514-398-2798
Oversight Committee
The oversight committee was established to help guide the MCF in prioritizing the development of new research capabilities and services that can be offered to investigators within McGill University and the broader scientific community. They are also tasked with yearly overview of the cost recovery pricing of MCF services as well as participating in method and technology development.
Director, Dr. Peter Siegel, and Co-Director, Dr. Lawrence Kazak help guide the metabolomics core facility by setting priorities as well as participate in technology development of the core.
Oversight Committee Members
Dr. Julie St-Pierre, University of Ottawa
Dr. Russell Jones, Van Andel Instintute
Dr. Michael Pollak, Lady Davis Institute
Dr. Dajana Vuckovic, Concordia University
Our Staff
Daina Avizonis, PhD, Facility Manager - Research Associate
Dr. Daina Avizonis obtained a Ph.D. in Chemistry in 1993 from the University of California (San Diego, USA). She has extensive expertise in NMR and mass spectrometry and is the head of the Metabolomics Core Facility established in 2009. She oversees daily operations of the core, participates in data collection, protocol development and is responsible for research project coordination with researchers and students.
Luc Choinière, Research Technician, GC/MS Specialist
Luc Choinière obtained a B.Sc. in Biology at McGill University (Montreal, Canada) and has over 30 years of experience in GC/MS and joined the Metabolomics Core Facility as a research technician in 2010. Luc maintains both GC/MS systems, helps develop new protocols and assists researchers collect and analyze GC/MS data.
Cian Monnin, Research Technician – LC-MS specialist
Cian Monnin obtained a M.Sc. in Biochemistry from Concordia University. While at Concordia he specialised in lipidomics and the associated sample preparation techniques. After obtaining his degree he worked at the PERFORM centre at Concordia University developing targeted methods for whole blood metabolic profiling and chiral separation of isomers. He joined the GCRC as a Technician September 2018.
Bozena Samborska, Research Assistant / Seahorse Technology
Bozena Samborska obtained a M.Sc. in Molecular and Cellular Biology in 2010 from the University of Guelph (Ontario, Canada). She is a Research Assistant and the Lab Manager in Dr. Kazak and works mainly in cancer metabolism. She is also responsible for Seahorse operation and training.
Mariana Russo, Research Technician – LC-MS specialist
Mariana Russo obtained M.Sc. in Analytical Chemistry from Concordia University. While at Concordia Mariana specialized in derivatization schemes to stabilize labile metabolites such as glutathione. She has extensive experience in LC-MS targeted profiling and stable isotope tracer analysis as well as experience volatile com
Our Services
Analytical Services
If you are new to working with the Metabolomics Core Facility, we strongly encourage you to read our sample preparation advice and recommendations.
The MIR is equipped with four mass spectrometers: two GC/MS, UPLC/QQQ and UPLC/QTOF. The facility staff also has access to the NMR spectrometers at the QANUC NMR facility and the MUHC-RI Platform for drug discovery. This allows the MIR to provide a wide array of analytical platforms for metabolite analysis. We offer both targeted metabolite and untargeted services.
In order to establish the best relationship possible with the investigators using the MIR, we ask you to review our “Terms of Use” document and provide us with a signed copy of the “Acknowledgment for conditions of use” document.
When you are ready to start a project with us, please read our General Recommendations if you have not worked in the area of metabolomics or metabolite profiling previously.
- Discussion with MIR Staff Member
- Cell/Tissue/Biofluid extraction protocol will be provided
- A pilot study on a few samples will be run for you free of charge to ensure
- ou are able to follow our extraction protocol for your specific project.
- Determine that the extraction protocol works for your specific project
- Ensure that the MIR can measure the metabolites of interest discussed in part #1
After a successful pilot study any minor changes or modifications to the protocols will be discussed. - A larger study will be designed, planned & scheduled.
It is imperative that we communicate well every step of the way for a successful metabolomics project!
Our services are always expanding. See our services below to learn more.
Targeted Analysis
Targeted analysis is performed to analyze or quantify specific metabolites. The instruments used for targeted analysis are NMR, GC/MS and LC-MS/MS. Targeted analysis depends on the following:
- The ability to obtain authentic or synthetic standards
- The ability to detect the standard (using any of the listed instruments)
- The ability to efficiently extract the endogenous compound(s) from the sample(s) and derivatize if necessary (GC/MS).
- The ability to have good chromatography (either GC or LC) of the endogenous compounds to cleanly separate them from other confounding metabolites.
We have attempted to group targeted metabolites into logical families. The families tend to be grouped either by metabolic pathway or chemical behavior or class. Note that there is overlap between groupings. When more than one analysis is requested, we do our best to try to analyze as much as possible from the given sample.
If you cannot find the metabolites you are interested in targeting, please make an appointment to talk with us. Our group has the expertise to create new methodologies specific to your needs. Custom analysis charges will apply (staff & instrument time) unless otherwise directed by the MCF oversight committee.
Amino Acids and Derivatives
|
alanine, beta-alanine |
glycine | phenylalanine |
|
allo-isoleucine |
histidine |
phospho-serine |
| arginine |
homocysteine |
proline |
| asparagine | homocystine | pyroglutamate |
|
aspartic acid |
hydroxyproline |
sarcosine |
| betaine | hypotaurine | serine |
| citrulline | isoleucine | taurine |
| cysteine | leucine | threonine |
| cystine | lysine | tryptophan |
|
glutamic acid |
methionine | tyrosine |
| glutamine | ornithine | valine |
|
gamma-aminobutyric acid |
The MCF offers both free and total amino acid analysis either by NMR, GC/MS or LC/MS depending on other desired analyses. The current library of amino acid and amino acid derivatives are listed
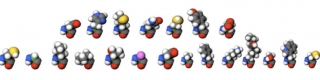
Citric Acid Cycle Intermediates
The citric acid cycle intermediates may be measured by LC/QQQ or GC/MS.
Note that oxaloacetate, pyruvate and a-ketoglutaric are not stable intermediates. For GC/MS analysis these intermediates are reduced using NaBD4 to their more stable alpha-hydroxy acids.
The intermediates are quantified by stable isotope dilution (ref: http://link.springer.com/article/10.1007/s11306-013-0521-1#page-1).
The following metabolites are quantified:
|
Lactic acid |
Alpha-ketoglutaric acid |
|
Pyruvic acid |
Succinic acid |
|
Citric acid |
Fumaric acid |
|
Isocitric acid |
Oxaloacetic acid |
|
Oxaloacetic acid* |
Malic acid |
|
2-hydroxyglutaric acid |
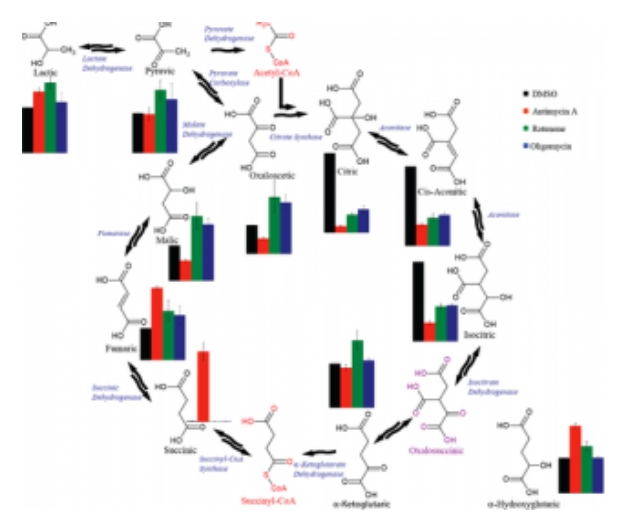
Free or Total Fatty Acids
Fatty acids generally vary in length from 4 to 28 carbons with various levels of unsaturation. Free fatty acids can be metabolized as a fuel (free fatty acids) or used as building blocks for cell walls, tri-glycerides and other lipids. Saponification allows for full fatty acid analysis compared to the generally lower level of free fatty acids.
The MCF provides GC/MS based untargeted analysis for fatty acids. Our fatty acid library is listed below. Other fatty acids of special interest can be added to our library assuming an authentic standard can be obtained.
Currently we provide ratios of fatty acid to an internal standard (D27-myristic). Absolute quantitation can be done by special request.
Current list of fatty acids in our library (more can be added!):
|
Montanic C28:0 |
Eicosapentaenoic C20:5 {{5,8,11,14,17}} |
Palmitic C16:0 |
|
Cerotic C26:0 |
Eicosapentaenoic C20:5 {{5,8,11,14,17}} Stearic C18:0 |
Palmitoleic C16:1 {{9}} |
|
Lignoceric C24:0 |
Oleic C18:1 {{9}} |
Palmitelaidic C16:1 {{t9}} |
|
Nervonic C24:1 {{15}} |
Petroselinic C18:1 {{6}} |
C15:0 |
|
Behenic C22:0 |
Vaccenic C18:1 {{11}} |
Myristic C14:0 |
|
Erucic C22:1 {{13}} |
Linoleic C18:2 {{9,12}} |
Myristoleic C14:1 {{9}} |
|
Arachidic C20:0 |
α-Linolenic C18:3 {{9,12,15}} |
C13 |
|
Eicosenoic C20:1 {{11}} |
γ-Linolenic C18:3 {{6,9,12}} |
Lauric C12:0 |
|
Eicosatrienoic C20:3 {{8,11,14}} |
Margaric C17:0 |
Capric C10:0 |
|
Arachidonic C20:4 {{5,8,11,14}} |
Caprylic C8:0 |
|
|
β-Hydroxy Acids |
||
|
Decanoic C10:0 3-OH |
Undecanoic C11:0 3-OH |
Lauric C12:0 3-OH |
|
Myristic C14:0 3-OH |
C15:0 3-OH |
Palmitic C16:0 3-OH |
Glycolytic Intermediates
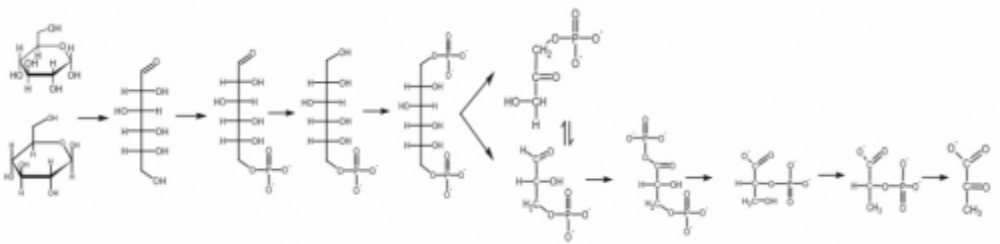
Glycolytic intermediates are measured by LC/QQQ and quantified by external calibration curves. Due to structural and molecular weight similarity it is not possible to resolve some intermediates.
Note also that glycolytic intermediates are normally found at very low concentrations, and therefore may be difficult to measure.
The intermediates in our protocol are as follows:
| Glucose |
Glyderaldahyde 3-phosphate |
|
Glucose 6-phosphate / Fructose 6-phosphate |
Dihydroxyacetone phosphate |
|
Fructose 1,6 bis-phosphate |
Phosphoenolpyruvate |
|
2-Phosphoglycerate |
Pyruvate |
|
3-Phosphoglycerate |
Lactate |
Nucleotide Analysis
The nucleotide analysis includes many mono, di and triphosphates, such as ATP, ADP and AMP for energy charge analysis.
This analysis also includes creatine and phosphocreatine since the ratio of these metabolites also contributes to the energy charge of the cell. Nucleotides are measured by LC/QQQ and quantified based on external standard calibration curves run at the same time as the samples of interest.
Our current library of nucleotides is listed below:
| Adenosine monophosphate (AMP) |
Inosine 5’ monophosphate (IMP) |
Flavin adenine dinucleotide (FAD) |
| Adenosine diphosphate (ADP) |
Inosine 5’ diphosphate (IDP) |
beta-Nicotinamide adenine dinucleotide (NAD+ ) |
| Adenosine triphosphate (ATP) |
Inosine 5’ triphosphate (ITP) |
beta-Nicotinamide adenine dinucleotide reduced (NADH) |
| Adeosine 3,5, cyclic monophosphate (cAMP) |
Cytidine 5’ monophosphate (CMP) |
beta-Nicotinamide adenine dinucleotide phosphate (NADP+ ) |
| Guanosine monophosphate (GMP) |
Cytidine 5’ diphosphate (CDP) |
beta-Nicotinamide adenine dinucleotide phosphate reduced (NADPH) |
| Guanosine diphosphate (GDP) |
Cytidine 5’ triphosphate (CTP) |
Uridine 5’-diphosphoglucose (UDP-glucose) |
| Guanosine triphosphate (GTP) |
Thymidine 5’ monophosphate (TMP) |
Uridine 5’- diphosphogalactose (UDP-galactose ) |
| Uridine 5’ monophosphate (UMP) |
Thymidine 5’ diphosphate (TDP) |
Uridine 5’- diphosphoglucuronic acid (UDP-glucuronic acid ) |
| Uridine 5’ diphosphate (UDP) |
Thymidine 5’ triphosphate (TTP) |
Uridine 5’ diphospho–N-acetylglucosamine (UDP-N-acetyl glucosamine) |
| Uridine 5’ triphosphate (UTP) |
We can add additional nucleotides upon request assuming authentic standards are available.
Nucleoside Analysis
The nucleoside analysis includes the nitrogenous bases, ribonucleosides, and deoxyribonucleosides. Nucleosides are measured by LC/QQQ and quantified based on external standard calibration curves run at the same time as the samples of interest.
Our current library of nucleosides is listed below. We can add additional nucleotises upon request assuming authentic standards are available.
| Adenine | Adenosine |
2'-Deoxyadenosine |
| Guanine | Guanosine |
2'-Deoxyguanosine |
| Cytosine | Cytidine |
2'-Deoxycytidine |
| Thymine |
5-Methyluridine |
2'-Deoxythymidine |
| Uracil | Uridine |
2'-Deoxyuridine |
Water Soluble Oxidative Stress Indicators
Please note that some metabolites listed here are redundant to the Amino Acids analysis. We attempt to capture reduced and oxidized forms of important metabolites involved in reducing oxidative stress.
These partially cover folate metabolism and the methionine cycle.
Taurine and hypotaurine can be easily added upon request.
|
Reduced Glutathione |
Cystathionine | Folate |
|
Oxidized Glutathione |
Homocysteine | Dehydrofolate |
|
Ascorbic Acid |
Methionine | Tetrahydrofolate |
|
Dehydroascorbic acid |
S-adenosylmethionine (SAM) |
|
| Cystine |
S-adenosylhomocysteine (SAH) |
|
| Cysteine |
gamma-glutamylcysteine |
Pentose Phosphate Pathway
The pentose phosphate pathway intermediates are quantified by LC/QQQ using external calibration curves. The following metabolites may be measured:
| Glucose | Ribose |
|
Glucose-6 phosphate |
Ribulose |
|
5-Phospho-D-Ribose 1 diphosphate |
Glucosaminic Acid |
|
6-Phosphogluconic acid |
Ribose 5-phosphate |
| Gluconate |
Ribulose 5-phosphate |
| Gluconolactone |
2-Deoxy-ribose 5-phosphate |
|
Sedoheptulose 7-phosphate |
2-Deoxyribose |
Untargeted Analysis
Due to the time commitment of untargeted analysis, the MCF currently only offers this service to GCRC members. We hope to offer this service more broadly in the near future.
Untargeted analysis will be managed as a close collaboration between the MCF and the investigator.
Three instruments can be used for untargeted analysis: LC/QTOF, GC/MS and NMR.
A general workflow is described below. This workflow is flexible and will be modified to meet the needs of the project.
Samples
Samples are extracted according to the chemistry of the metabolites that the scientist is most interested in (e.g. polar metabolites vs fatty acids).
Data acquisition
- NMR: Samples are prepared and data are acquired
- GC/MS: samples are derivatized (usually MOX/TMS or MOX/TBDMS) and GC/MS data collected
- LC/MS: an appropriate column and buffer conditions are chosen depending on the class of molecules of interest (e.g. organic acid vs nucleotide) and LC/QTOF data are acquired.
Data alignment or recursive analysis
- NMR data are fit using Chenomx software or other software as defined by the project.
- GC/MS data are deconvoluted by chromatography and retention time using AMDIS or MassHunter Qualitative software (Agilent).
- LC/QTOF data are processed for molecular features including all ions (plus their isotopes) and adducts. This may be repeated in a recursive workflow using MassHunter Qualitative software and Mass Profiler Professional (MPP) (Agilent).
Statistical analysis
- NMR assigned metabolites, bins, or modeled peaks are subjected to statistical analysis (PCA, PLSDA, etc) using MPP (Agilent) or MetaboAnalyst. Metabolites or peaks causing the differentiation between groups are identified.
- GC/MS spectra are tentatively identified using Feihn, Bains (Steadman Metabolism laboratory, Duke University) or NIST11 databases. The identified and unidentified metabolites are subjected to data alignment across different samples and statistical analysis using MPP. Identified and unidentified metabolites that cause differentiation between groups are “tagged” for further identification.
- LC/QTOF extracted metabolites or “features” are subjected to data alignment across different samples and statistical analysis using MPP. Differentiating “features” are “tagged” for identification.
Metabolite identification
- NMR : metabolites identified using 1D and 2D NMR techniques. Significant metabolites may be spiked into samples to confirm chemical shift, spectral patterns and a quantitative increase in the metabolite’s concentration. The HMDB , MMSD and BMRDB are used to help identify metabolites and their spectra.
- GC/MS based metabolites are identified and ideally new samples are split where one is spiked with the authentic metabolite standard(s) confirming an increase in the putatively identified metabolite and confirmation of mass spectrum and retention time.
- LC/MS: samples are re-run where the statistically significant differentiating metabolites are targeted for MS/MS analysis using different collision energies (Product ion scanning). These data are then used to identify the metabolites (tentative). The METLIN accurate mass and MS/MS database as well as the Human Metabolome Database are used to help the identification. If possible, samples are spiked with authentic metabolite standards to confirm an increase in the significant metabolite peak intensity as well as to confirm retention time, mass spectrum and fragmentation pattern.
Validation
Once metabolites have been identified, the pathways where they are up-regulated and down-regulated need to be elucidated. Using Pathway Analyst (Agilent), Cytoscape (NRNB), MetPA (University of Alberta) etc, can facilitate the identification of active pathways. This data can be compared to other «omics» data (transcription, protein levels etc). Further analyses such as pathway inhibitors, genetic manipulations and flux analysis are encouraged to confirm pathway use and directionality.
GC-MS Systems
The Agilent 5975C Series GC/MSD with the Triple-Axis Detector GC-MS systems are used for targeted (SIM), untargeted and flux analysis experiments. Data are collected and analyzed by Chemstation software or Mass Hunter and Mass Profiler professional is used for untargeted differential analysis. Spectra are identified using Fiehn, Bains (Steadman Metabolism laboratory, Duke University) or NIST17 databases and authentic samples.
LC-MS Systems
The Agilent 6470 Triple quadrupole LC-MS System are designed to be compatible with ultra-high pressure liquid chromatography (UHPLC, 1290 Infinity LC System) separation for fast targeted analysis. The Multiple Reaction Monitoring strategy allows targeting of hundreds of compounds in a single run for accurate measurements. It is dedicated to low molecular-weight compounds and biomolecules analysis. We use the MassHunter software series for data acquisition, identification, and quantitation.
The Agilent 6540 UHD Accurate-Mass Q-TOF LC-MS system is designed to be compatible with ultra-high pressure liquid chromatography (UHPLC, 1290 Infinity LC System) separation for fast and high confidence untargeted analysis of complex samples. It offers high dynamic range quantification as well as ultra-high-definition mass accuracy and mass resolution for MS and MS/MS data. We use the Agilent Jet Stream thermal-gradient-focusing technology to provide exceptional MS/MS sensitivity and identification of trace-level compounds.
Agilent 6545 UHD Accurate-Mass QTOF SFC/LC-MS system is designed to work either with super critical fluid chromatography or liquid chromatography. This is instrument, similar to the 6540, adds an orthogonal separation technique for the facility.
Walk-up Media Analyzer
The BioProfile 400 analyzer is a user-friendly instrument that can be used to quickly (3 min/sample) measure glucose, lactate, glutamine, glutamate, ammonium, sodium and potassium concentrations as well as pH from tissue culture media or serum/plasma. For further information about this instrument and measurable concentration ranges, please visit the manufacturer’s website:
http://www.novabiomedical.com/products/bioprofile-analyzer/
The BioProfile 400 is located on the 7th floor of the McIntyre building in room 726.
To use the BioProvile 400, the researcher must first make an appointment with a core staff member for a training session that lasts about 30 minutes.
Sample requirements:
A minimum media volume of one milliliter is necessary for the proper use of this instrument. Media samples must be spun down to remove any cellular debris prior to analysis.
NMR
- QANUC 500 MHz NMR
- QANUC 800 MHz NMR
The staff of the MCF has access to the QANUC NMR facility and the MUHC-RI drug discovery platform systems which house 800MHz, 600MHz, 500MHz systems equiped with cryogenically cooled probes for improved sensitivity.
Cellular Respiration - Seahorse Instruments
The facility has three seahorse instruments: XFe24 (24 well format), XFe96 (96 well format) and Xfp (8 well format). Using transient micro-chamber technology, the Seahorse instruments can measure the rate of oxygen consumption and the extracellular acidification rate of tissue culture cells (both adherent and suspension) using a specialized bicarbonate-free media system. The cellular system can then be perturbed by the addition of nutrients, drugs or mitotoxins to measure changes in the rate of oxygen consumption (respiration) and the rate of acidification (glycolysis).
The Seahorse instruments are located on the 7th floor of the McIntyre building in room 726.
Use of the Seahorse instruments requires approximately two days of training. The training will include instruction on how to calibrate the dosage of various mitotoxins (if desired) and general use of the instrument. An experienced trainer will be assigned to you.
Sample requirements:
Use of the Seahorse instruments does require tissue culture and growing cells using specialized media. If possible, tissue culture work should be done at the researcher’s site to prevent cross contamination in incubators & hoods. If this is not possible, other arrangements can be made.
If you have any question or special request, please contact us by filling the form below.
The Goodman Cancer Research Centre's metabolomics core facility consists of a large array of platforms managed in cooperation with the Metabolism and Cancer laboratories and with the guidance of the oversight committee. A list of selected publications for the MIR can be found here.