At a glance
Detecting cancer early, before it has progressed to treatment-refractory, metastatic disease, is vital for improving patient outcomes. But to capitalize on early detection, it is necessary to predict which early-stage tumours will become aggressive and develop therapeutic strategies to intervene in this process. This requires a new generation of biomarkers – molecules, genetic changes, or other measurable indicators – that can inform on the likelihood that early-stage cancers will progress and whether they will respond to therapies. It also necessitates a deep understanding of the molecular and cellular changes that drive tumourigenesis and cause progression, laying the foundations for a new generation of curative therapies.
By using our state-of-the-art technology and collaborating with our clinical partners, we have developed a world-leading program to discover how early cancer cells and their microenvironments evolve to become aggressive, predict which early cancers are high-risk, and develop biomarkers and precision medicine strategies to match patients with therapies that have curative potential, including immunotherapies. This strategy will allow us to take advantage of improvements in early cancer detection by optimizing treatment to intercept high-risk cancers before they progress.
Areas of Focus
Team members
Our Discoveries
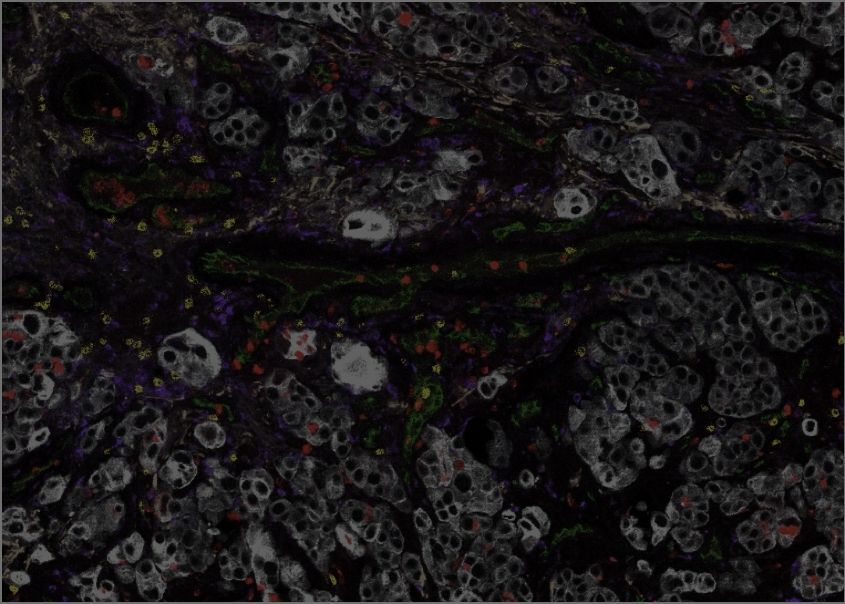
We have developed a machine learning platform, incorporating an advanced AI-based form of image analysis known as computer vision, to study large datasets generated using our single-cell, spatial “-omics” technology. Working with collaborators at the MUHC, the TFRI Marathon of Hope Cancer Centres Network, the Quebec Cancer Consortium, and the McGill Lung Cancer Research Network, we have used this technology to deliver a wealth of new information on how the immune microenvironment is organized within lung cancers. This has led to new biomarkers that predict which lung cancers will recur following surgery, and which patients with recurrent lung cancer will respond best to immunotherapy. The GCI is a major scientific partner of one of the world’s most important trials of immunotherapy in early lung cancer, in the pre-surgery (“neoadjuvant”) setting. This partnership allows our unique technology and analysis platforms to pinpoint the features of the tumour immune microenvironment that predict the response of early-stage lung cancers to this treatment.
Forde P.M., Spicer, J., CheckMate 816 Investigators. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. New England Journal of Medicine. 2022 May 26;386(21):1973-1985.
Karimi, E., et al. Machine learning meets classical computer vision for accurate cell identification. bioRxiv 2022.02.27.482183
Sorin, M., et al., Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature vol. 614, pp. 548–554 (2023).
Sorin, M. et al., Single-cell spatial landscape of immunotherapy response reveals mechanisms of CXCL13 enhanced antitumor immunity. J Immunother Cancer. 2023 Feb;11(2):e005545. doi: 10.1136/jitc-2022-005545. PMID: 36725085
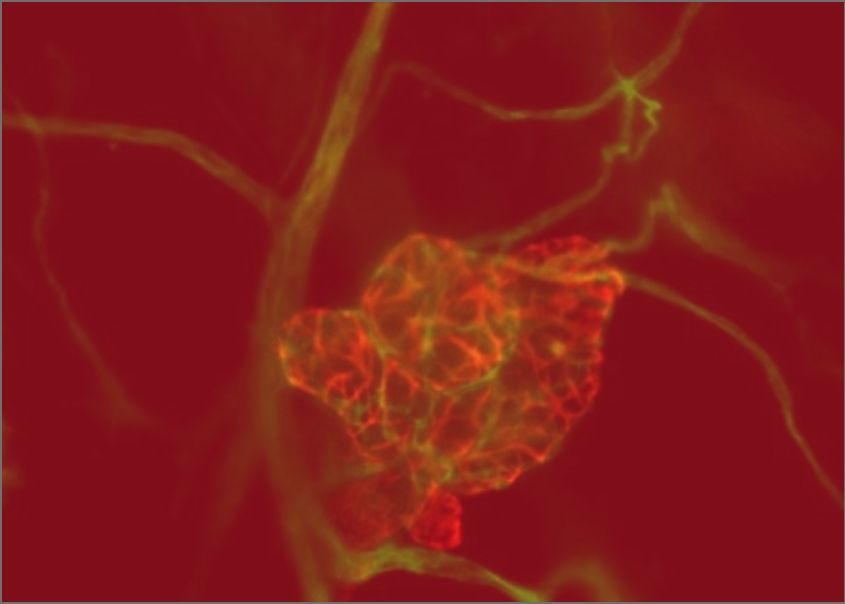
A deeper understanding of the earliest stages of tumour formation is needed to detect and treat cancers while they are still manageable. GCI scientists have used innovative models and single-cell analysis to reveal the cell of origin and key genetic programs of the most common type of ovarian cancer. Their work has expanded our fundamental knowledge of how reproductive tract develops, while pointing the way to new treatments for ovarian cancer, a highly aggressive disease with limited therapeutic options.
Teng, K., et al. Modeling High-Grade Serous Ovarian Carcinoma Using a Combination of In Vivo Fallopian Tube Electroporation and CRISPR-Cas9-Mediated Genome Editing. Cancer Research. 2021 Oct 15;81(20):5147-5160.
Ford, M.J., et al. Oviduct epithelial cells constitute two developmentally distinct lineages that are spatially separated along the distal-proximal axis. Cell Reports. 2021 Sep 7;36(10):109677.
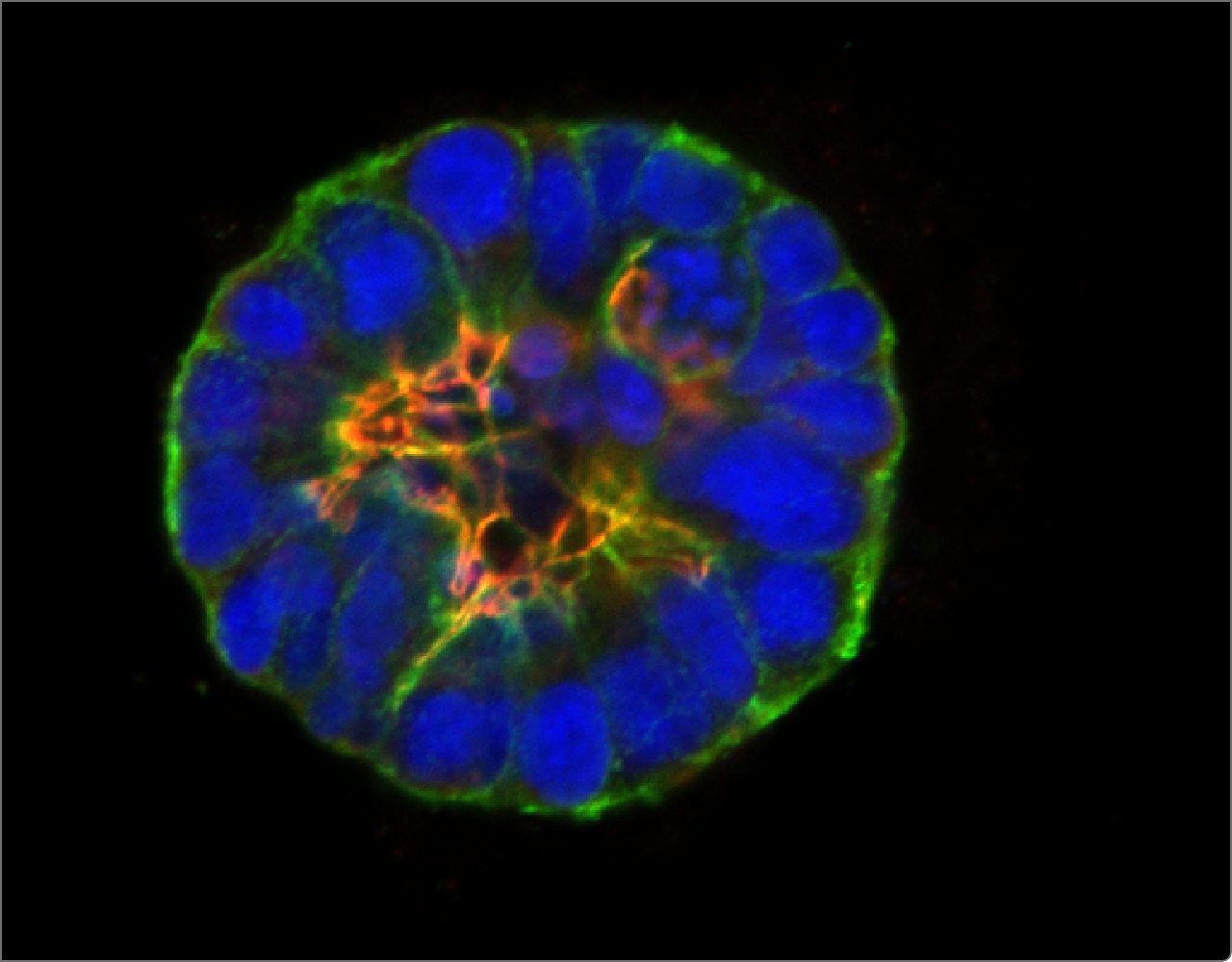
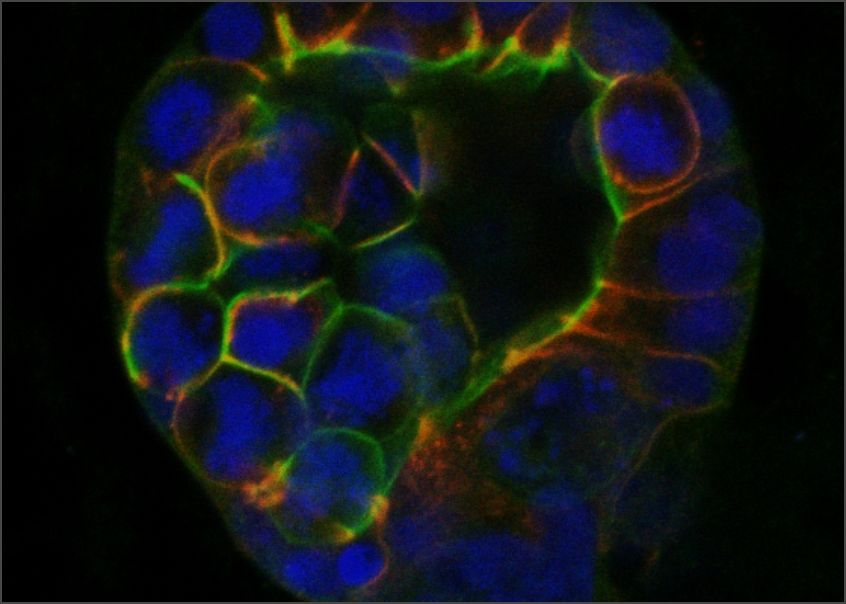
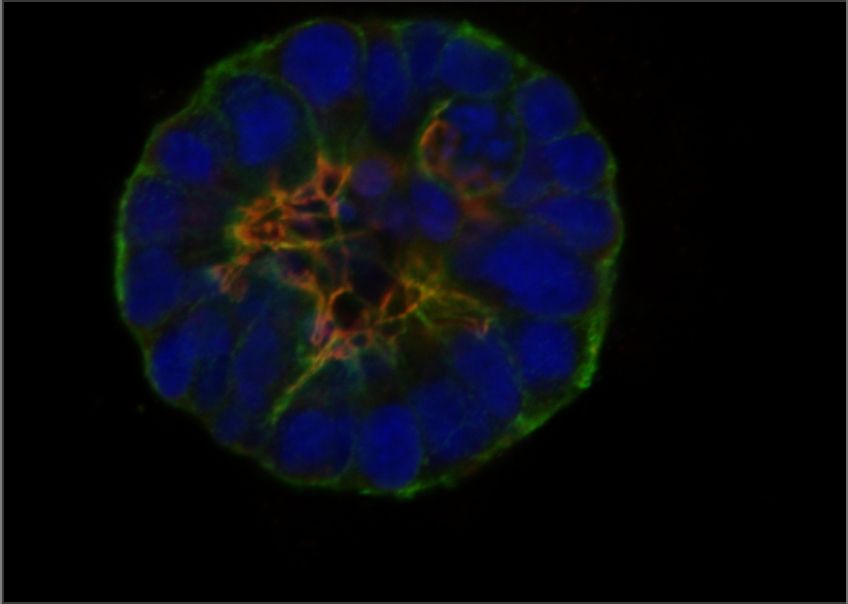
Cells organize their internal structure and their interactions with each other during normal tissue development, but this organization (referred to as epithelial polarity) is lost in many cancers. GCI investigators have been instrumental in establishing that the early steps of breast cancer development involve loss of epithelial polarity and in revealing the molecular mechanisms involved.
McCaffrey, L.M., et al. Loss of the Par3 Polarity Protein Promotes Breast Tumorigenesis and Metastasis. Cancer Cell. 2016 Aug 8;30(2):351-352.
Halaoui, R. , et al. Progressive polarity loss and luminal collapse disrupt tissue organization in carcinoma. Genes & Development. 2017 Aug 1;31(15):1573-1587.
Wang, L.-T., et al. Rajah A, Brown CM, McCaffrey L. CD13 orients the apical-basal polarity axis necessary for lumen formation. Nat Commun. 2021 Aug 4;12(1):4697.
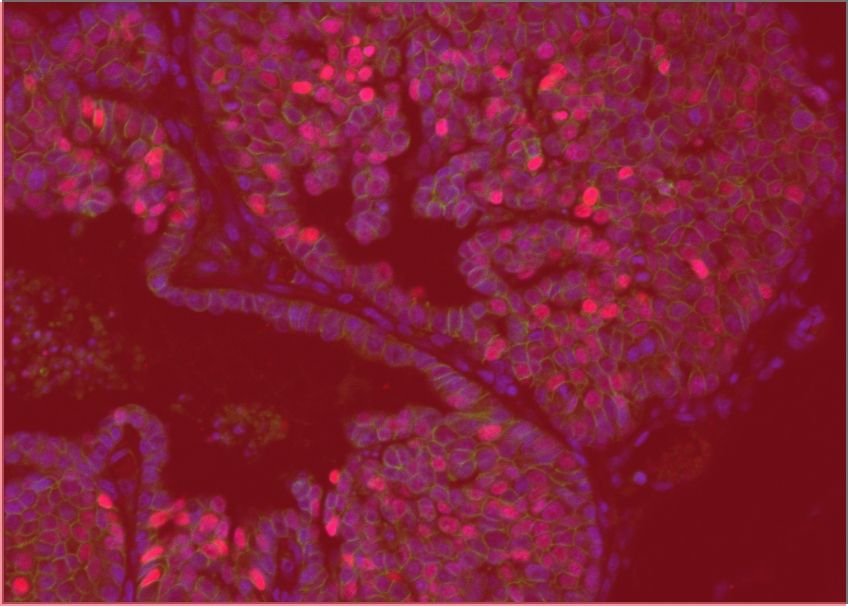
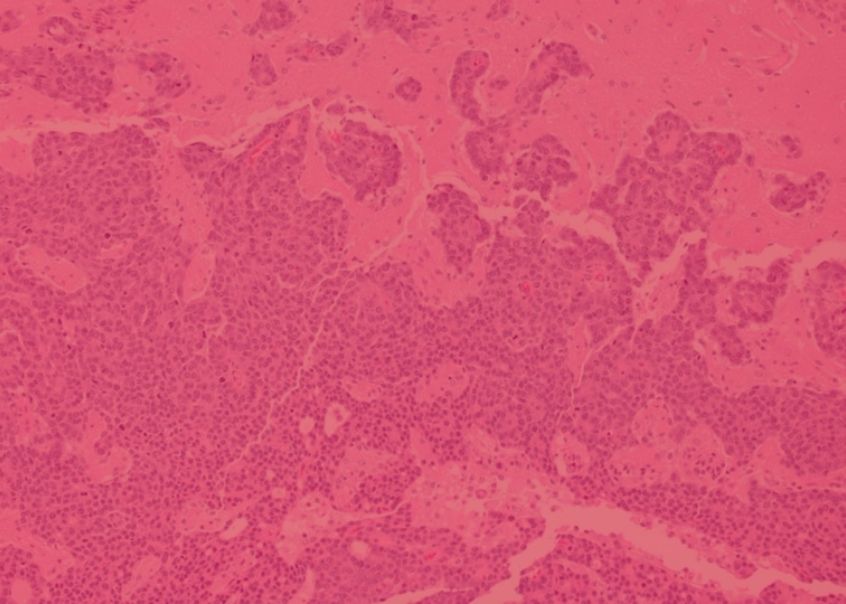
Cancer diagnosis relies heavily on analyzing the microscopic anatomy of tumour biopsies, a process known as histology. This analysis is typically performed manually by pathologists, but computer-aided techniques are advancing and are poised to increase the speed and accuracy of cancer diagnosis. GCI scientists are working with experts in artificial intelligence to develop methods of analyzing routine histological images using a technique known as deep learning. Their work is improving the efficiency of automated, AI-assisted detection of early cancers and has shown promise in samples of breast and colon cancer, two tumour types where histological analysis of biopsies remains the gold standard for diagnosis.
Belharbi, S. et al. Deep Interpretable Classification and Weakly-Supervised Segmentation of Histology Images via Max-Min Uncertainty. IEEE Trans Med Imaging 2022 Mar;41(3):702-714. doi: 10.1109/TMI.2021.3123461